GlucoMen Day CGM Sensor
The Electrochemical Sensor measures the glucose level present in the interstitial fluid for 14 days. Data is stored in the Transmitter and automatically sent via Bluetooth to the dedicated App, which displays a new real-time glucose value every minute.

Gentle
on the skin
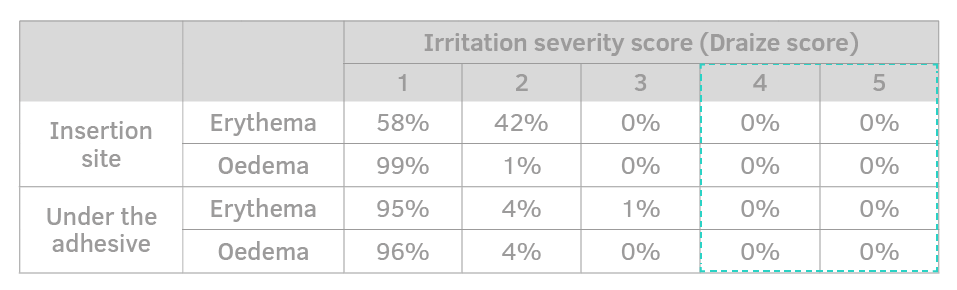
The larger patch surface and the low glue concentration minimise the chances of erythema and skin sensitisation.
Adherence with skin is well distributed to avoid involuntary movements and to better withstand shocks.
- Painless
- No adverse events

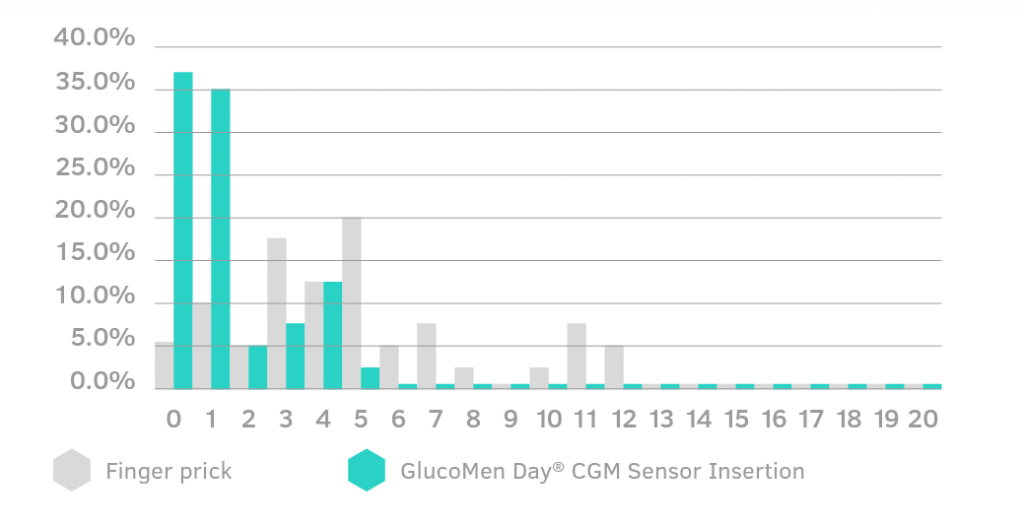
Sensor Insertion Pain Assessment
Pain from a GlucoMen Day CGM Sensor insertion has been reported to be on average 1.3 over a pain scale of 0-20. For comparison, finger pricking had an average score of 4.9.
(1) GlucoMen Day CGM Technical File, CEVAL Study.
Light
and Small

Transmitter

sensor patch
The GlucoMen Day CGM consists of a rechargeable transmitter and a sensor patch.
The GlucoMen Day CGM Sensor weighs only 25 g and is only 7 mm thick.