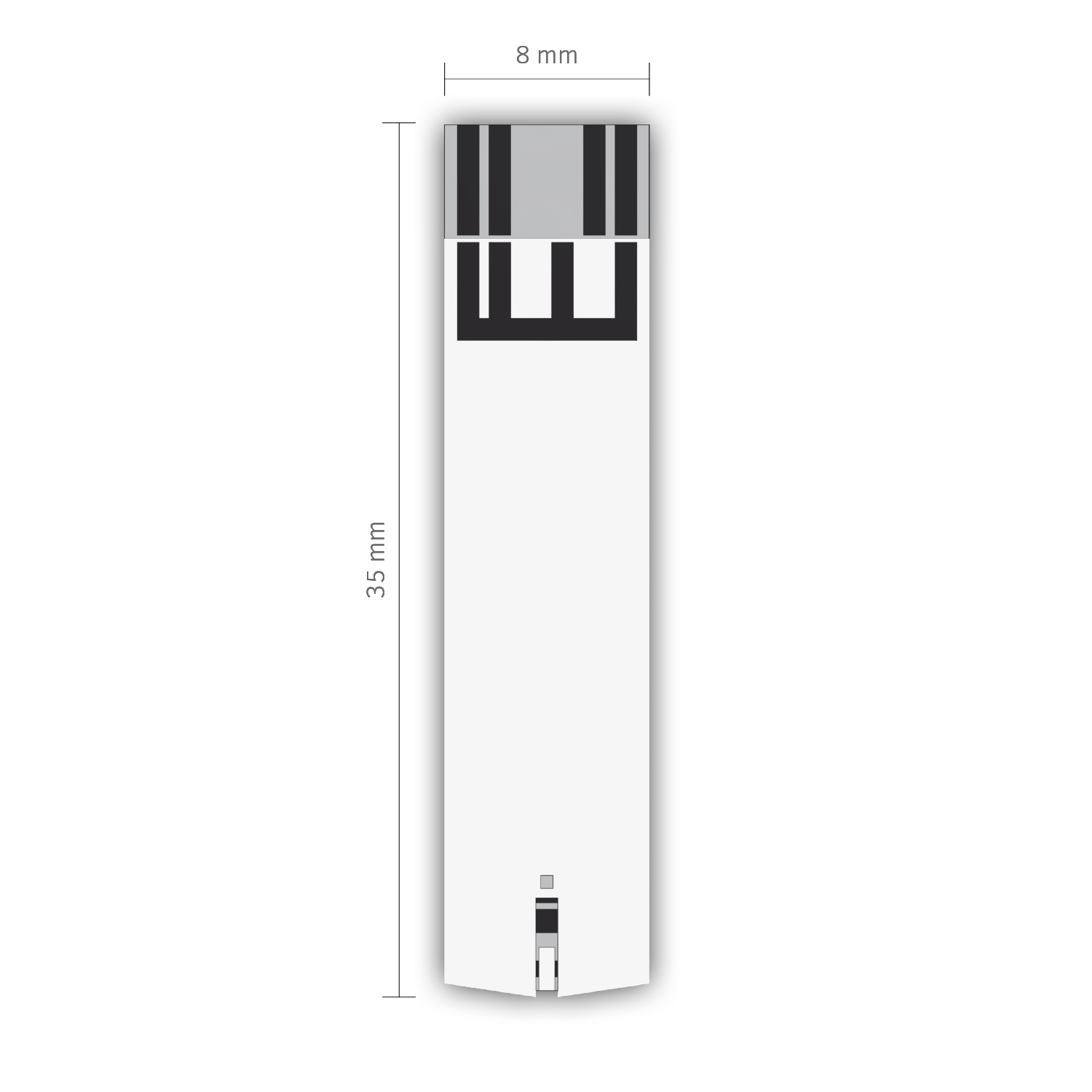
glucose + ketones test strips
Accuracy & Ease of use
for people with diabetes

- high analytical performance even for values below 60 mg/dL, which are critical for the detection of hypoglycemic episodes
10/10 accuracy
-
performance that surpasses
ISO standards 1

- analytical accuracy 2
- clinical accuracy 2
- reduced possibility of error in hypo detection
figure adapted from Ref. 3
high precision
- high repeatability performance, even in critical intervals for hypoglycemia
In hypoglycemia, repeatability of measurements is important because the effect of treatment may only be temporary, so blood glucose should be measured every 15 minutes 4
high hematocrit
compensation
- meets the ISO standards in the range of hematocrit 10-70% 6
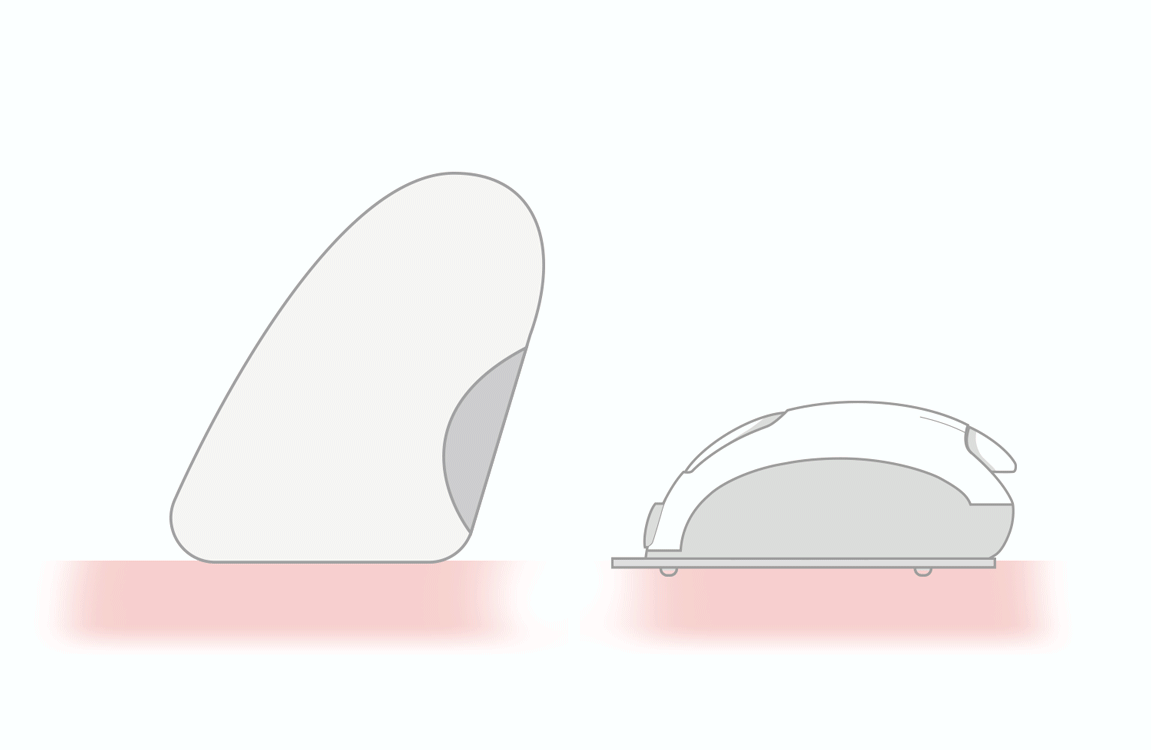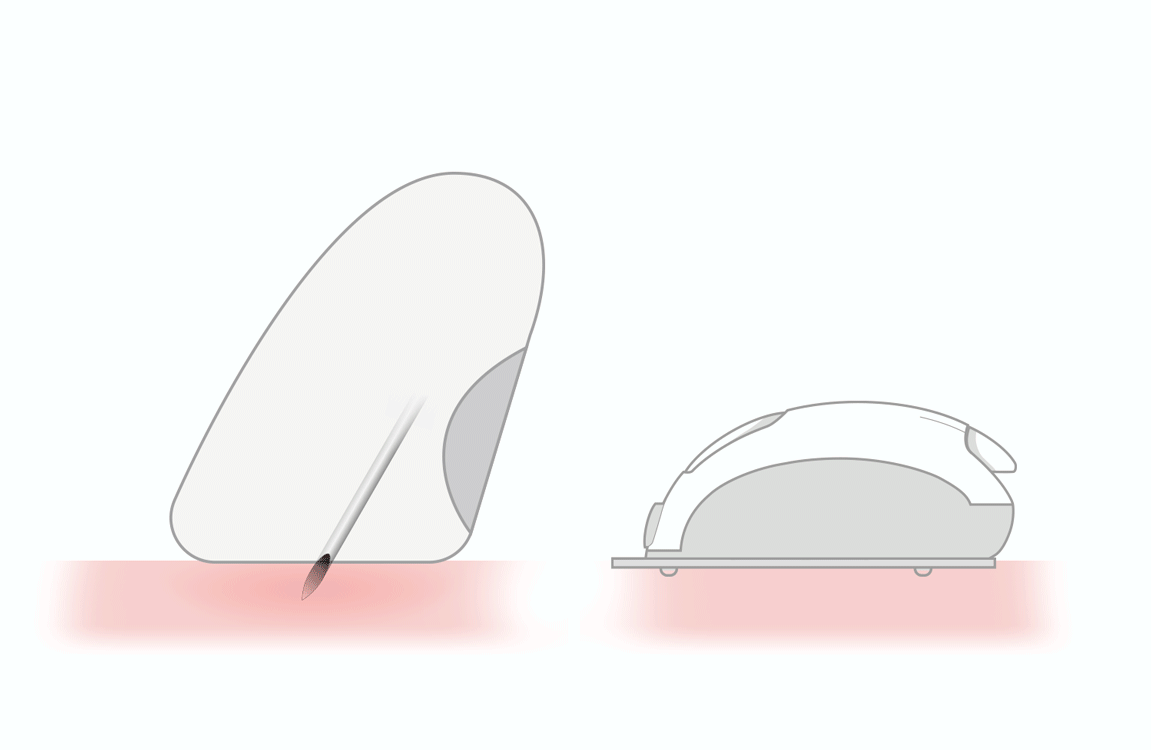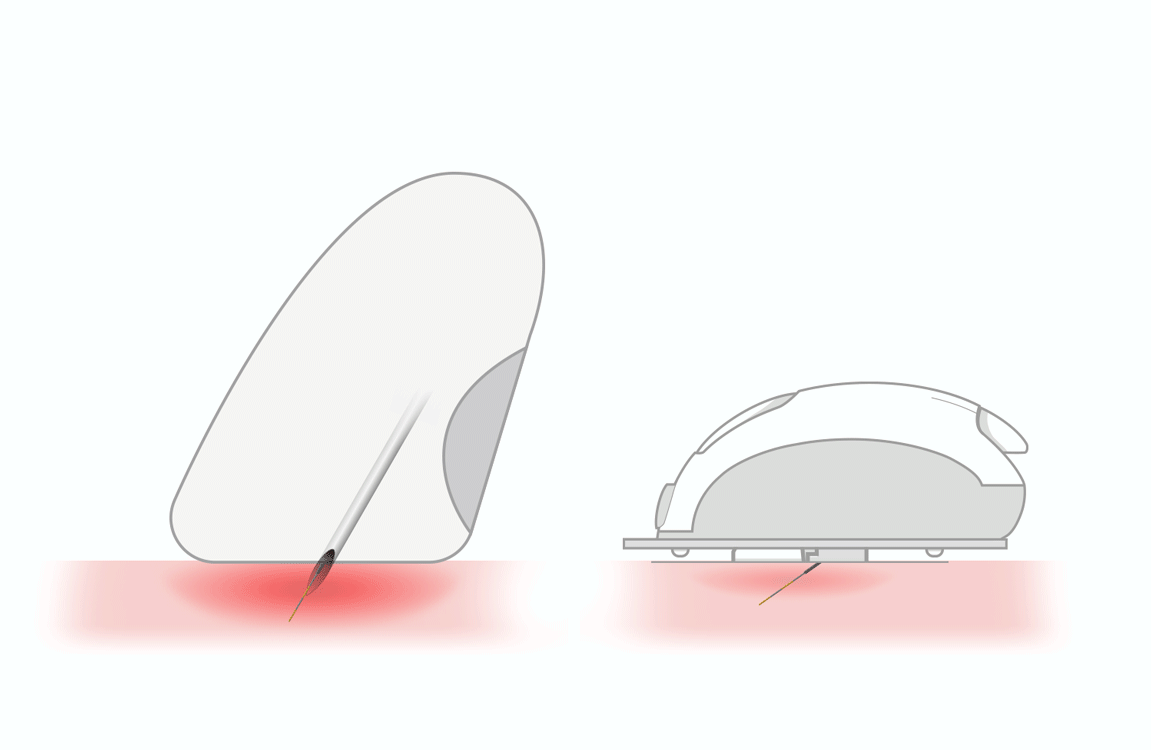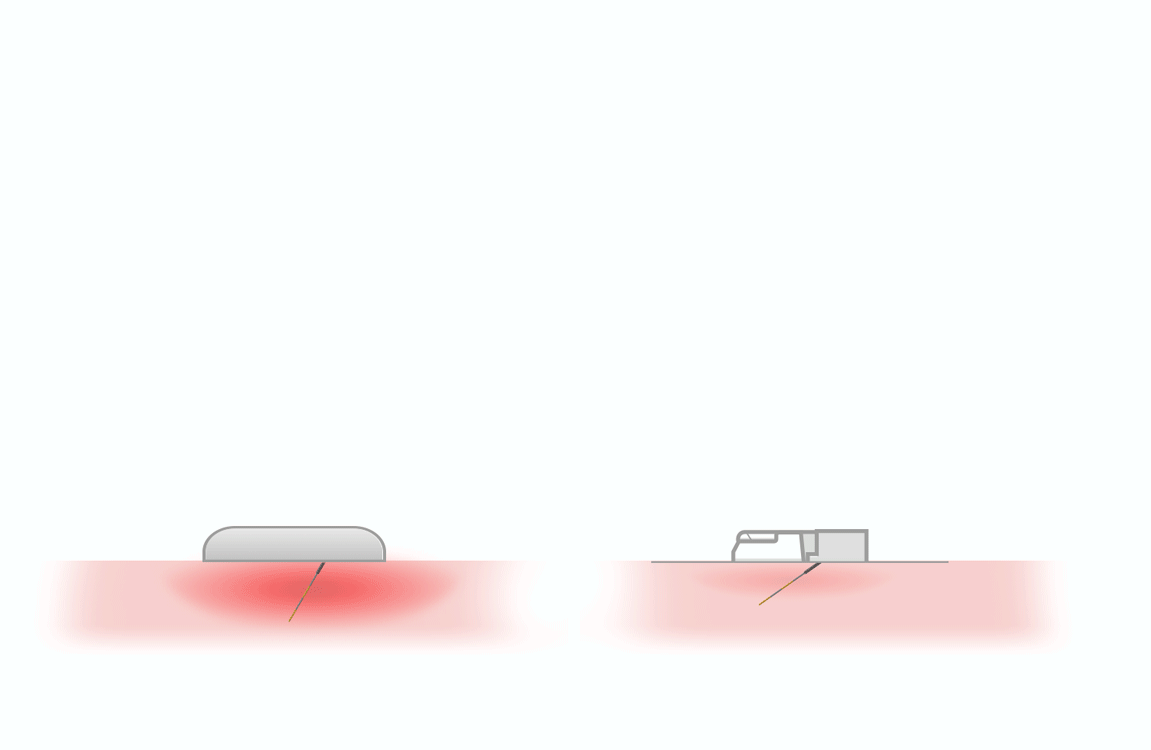
WIDE STRONG EASY TO HANDLE
Insertion Zone
Reaction Zone
easy to use
- wide and strong strips for easy handling
- user friendly insertion
- quick tests: 5 seconds for glucose, 8 seconds for ketones
- reduced blood sample volume: 0.5 μL for glucose, 0.8 μL for ketone

Test strips for self-monitoring of blood glucose and ß-Ketone with meters of the GlucoMen areo line
Technical Specifications
GlucoMen areo Sensor
GlucoMen areo ß-Ketone Sensor

Unit of measurement: mg/dL – mmol/L
Test range: 20-600 mg/dL (1.1–33.3 mmol/L)
Hematocrit range: 10-70% (compensated hematocrit)
Strips: GlucoMen areo Sensor
Sample volume: 0.5 μL
Analysis duration: 5 seconds
Test methodology: Electrochemical method based on glucose oxidase (GOD, from Aspergillus niger). Mediator: hexacyanoferrate (III) ion
Storage conditions of the strips: Temperature: 4-30°C | Relative humidity: 20-90%
Expiry of strips: 24 months for an unopened package; 12 months from opening the bottle
Unit of measure: mmol/L
Test range: 0.1-8.0 mmol/L
Hematocrit range: 20-60% (compensated hematocrit)
Strips: GlucoMen areo ß-Ketone Sensor
Sample volume: 0.8 μL
Analysis duration: 8 seconds
Test methodology: Electrochemical method based on ß-hydroxybutyrate dehydrogenase. Mediator: 1,10-phenanthroline-5,6-dione
Storage conditions of the strips: Temperature: 4-30°C
Expiry of strips: strips are individually packed in aluminium blisters, valid for 18 months from the date of manufacturing.
REFERENCES
1. EN ISO 15197:2015 – In vitro diagnostic test systems – Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus.
2. Accuracy Evaluation of the GlucoMen areo blood glucose monitoring system. Schmidt Group Practice Clinic (Hsinchu, Taiwan). Study performed referring to EN ISO 15197:2015, Par.6.3, as part of the CE registration Technical File. A. Menarini Diagnostics (Florence, Italy). Data on file.
3. Breton MD, Kovatchev BP. Impact of Blood Glucose Self-Monitoring Errors on Glucose Variability, Risk for Hypoglycemia, and Average Glucose Control in Type 1 Diabetes: An In Silico Study. J Diabetes Sci Technol 2010; 4(3): 562-70.
4. Slama G, Traynard PY, Desplanque N, Pudar H, Dhunputh I, Letanoux M, Bornet FR, Tchobroutsky G. The search for an optimized treatment of hypoglycemia. Carbohydrates in tablets, solutin, or gel for the correction of insulin reactions. Arch Intern Med. 1990 Mar;150(3):589-93. PMID: 2310277.
5. Repeatability evaluation according to ISO15197:2013, section 6.2.3. (part of GlucoMen areo Technical File).
6. Hematocrit Compensation of the GlucoMen areo blood glucose monitoring system. Study performed referring to EN ISO 15197:2015, Par.6.3, as part of the CE registration Technical File. A. Menarini Diagnostics (Florence, Italy). Data on file.