advanced lancets for finger pricking*
Comfort & Safety
for people with diabetes

sensitive and
smooth pricking 1
- thin lancet (33 gauge)
- triple sharpened tip
- polished stainless steel

safe to use
- sterilized using irradiation
- sterile needle protected by cap
- QMS compliant with EN ISO 13485:2016

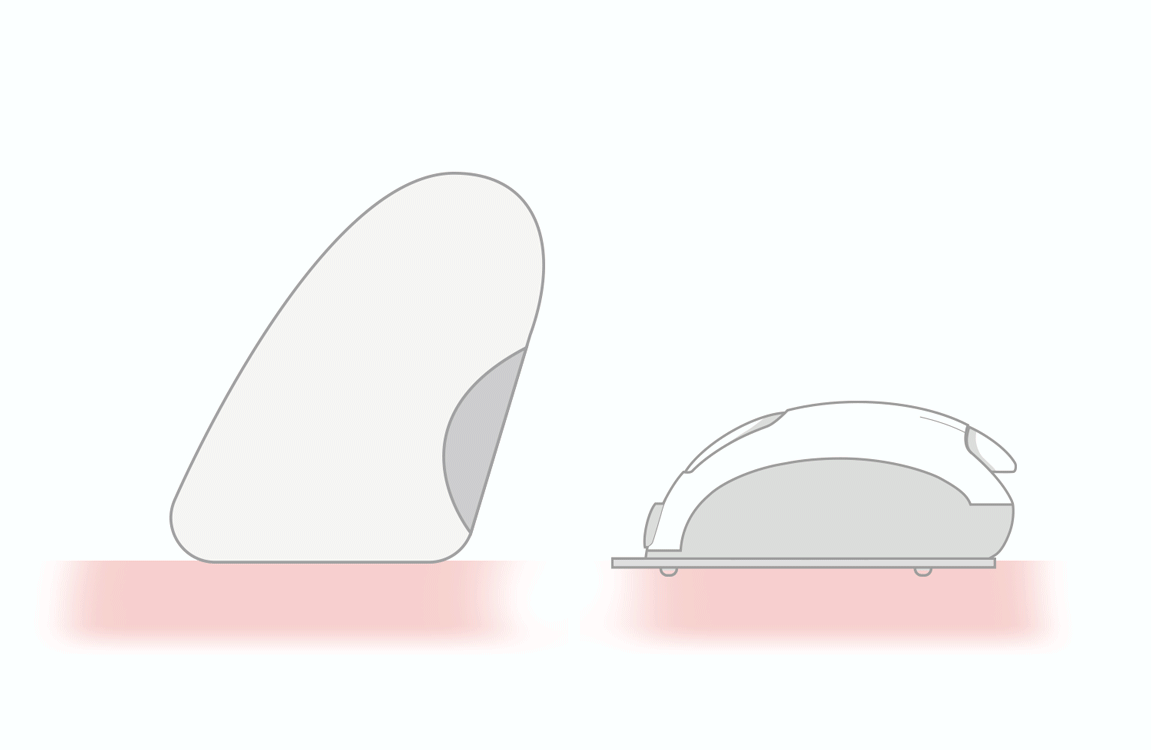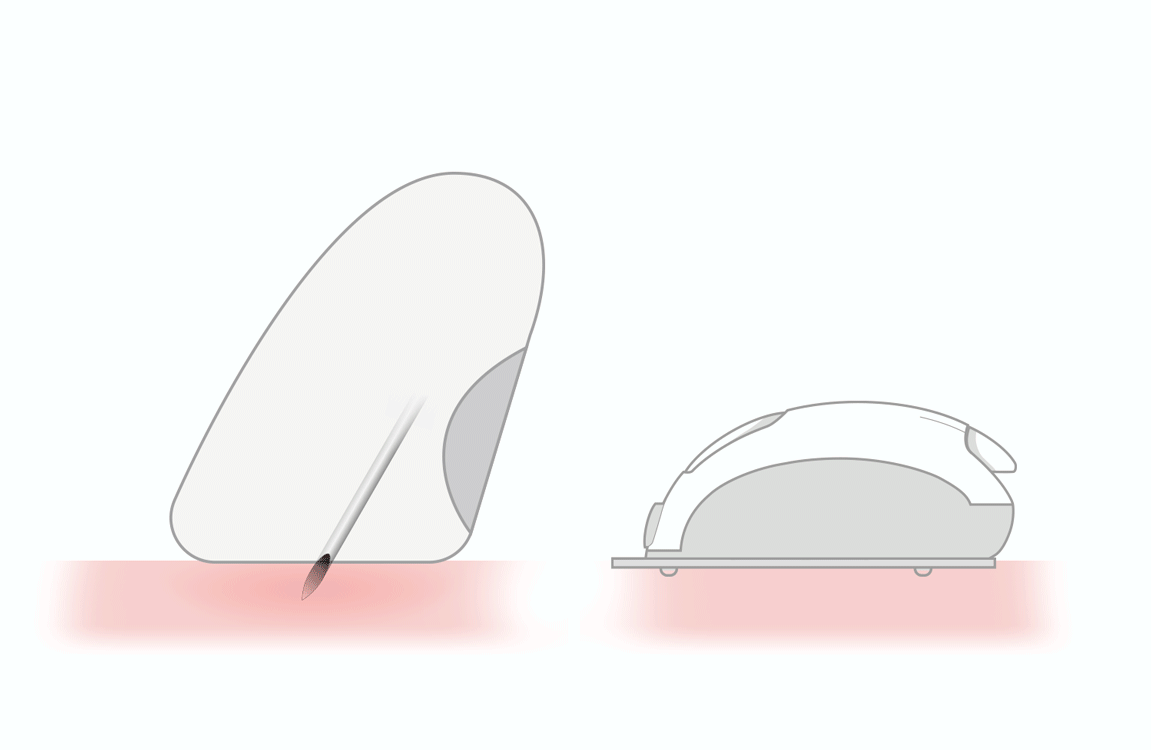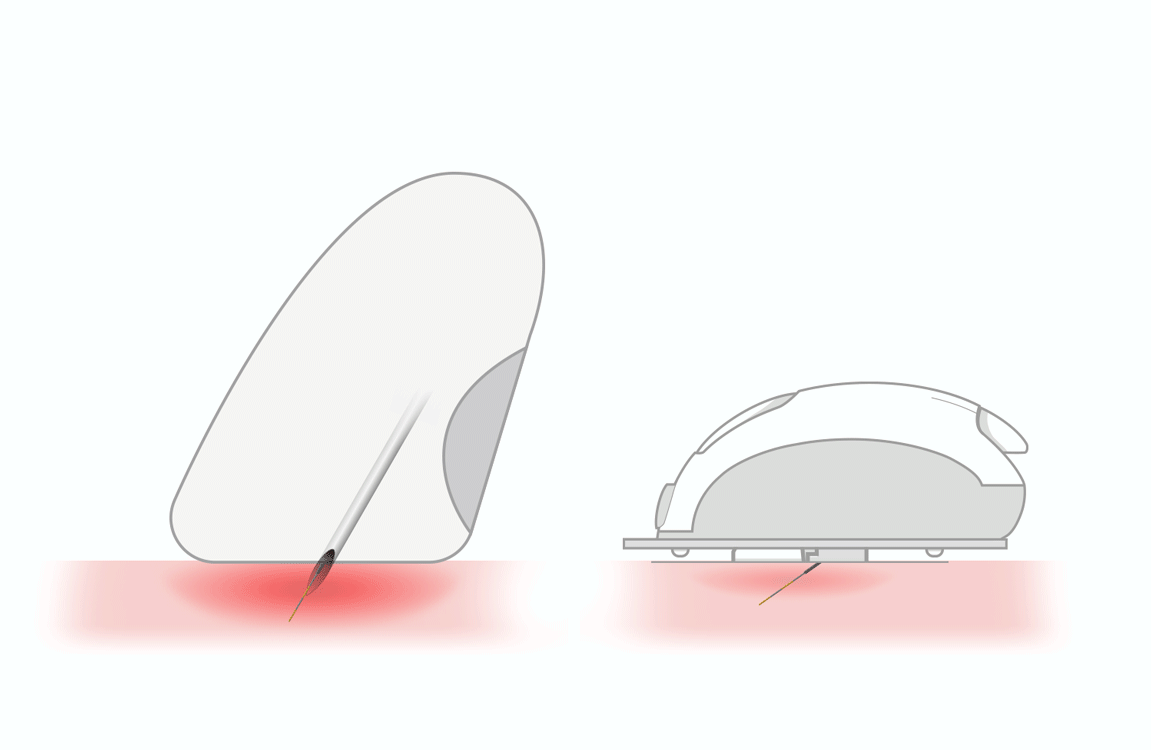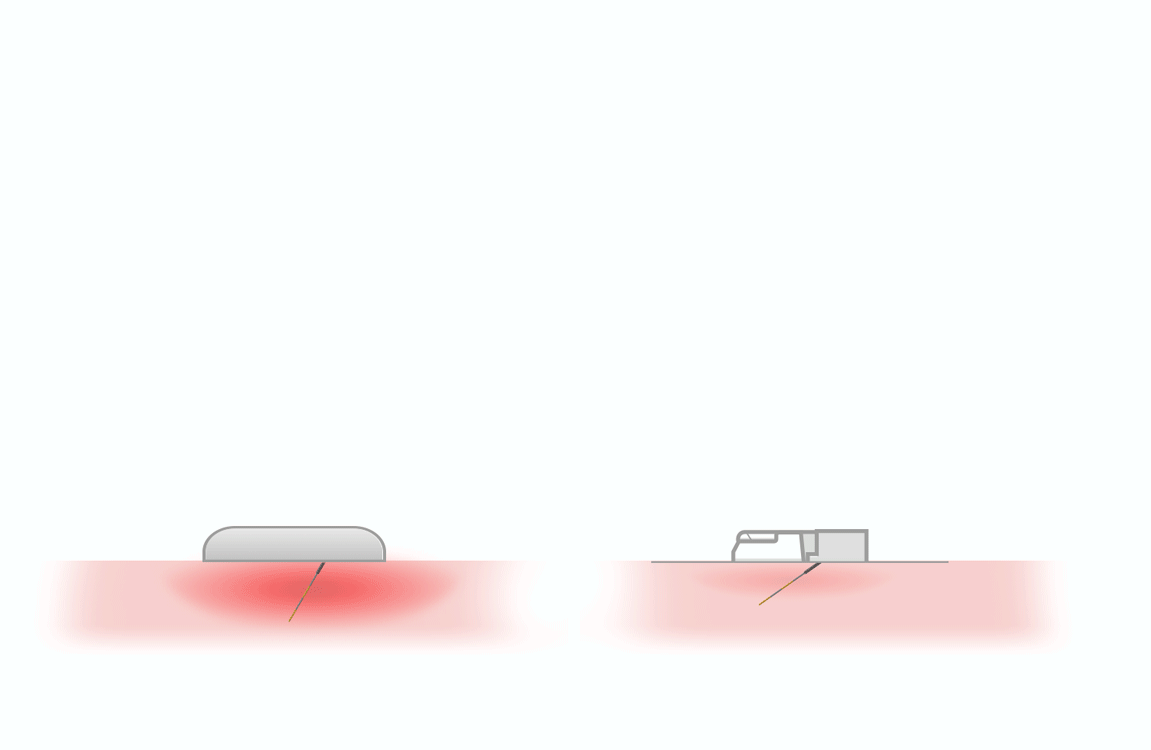
PROTECTIVE CAP
BODY LANCET

universal design
- compatibility with most lancing devices 2

- 5 adjustment levels
-
AST cap
for Alternative Site Testing - safe lancet ejector


Technical Specifications
Glucoject Lancets PLUS

* Sterile, single-use medical devices
intended to be used with a lancing device by lay
users for capillary blood sampling
Glucoject Lancets PLUS lancets are sterile, single-use medical devices intended to be used with a lancing device by lay users for capillary blood sampling.
The lancets consist of the following parts:
• needle (lancet) made of stainless steel, for medical use
• lancet body
• protective cap

5 years from sterilization date.
The lancet remains sterile until the protective cap is removed.
• Does not contain natural latex
• Does not contain PVC
• Does not contain phthalates
• Does not contain benzene
REFERENCES
1. Kocher S, Tshiananga JK, Koubek R. Comparison of lancing devices for self-monitoring of blood glucose regarding lancing pain. J Diabetes Sci Technol. 2009 Sep 1;3(5):1136-43. doi: 10.1177/193229680900300517. PMID: 20144427; PMCID: PMC2769891.
2. Data on file – List of lancing devices work with Glucoject Lancet PLUS personal lancets type 560 manufactured by HTL-Strefa