thanks to lower
penetration force 1, 2
- triple sharpening
- smooth silicone lubrication along the entire length of the needle
- electropolished needle tip
maximizes flow rate and reduces injection time 3
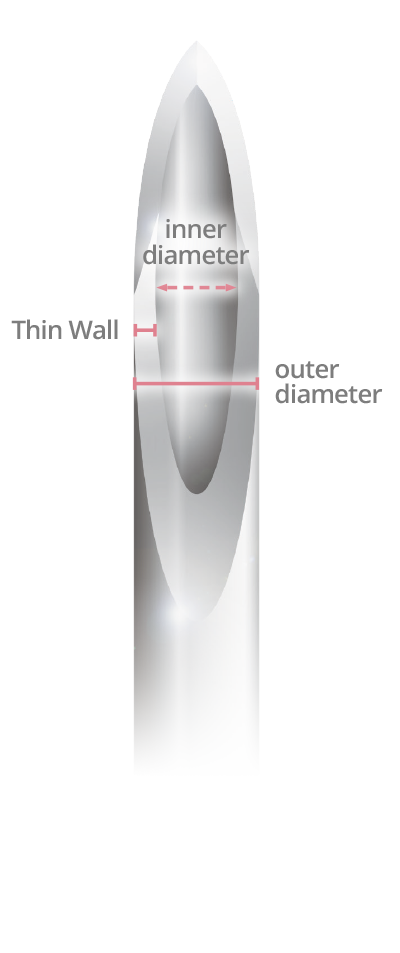
Same outer diameter, larger inner diameter than regular wall.
- Thin Wall
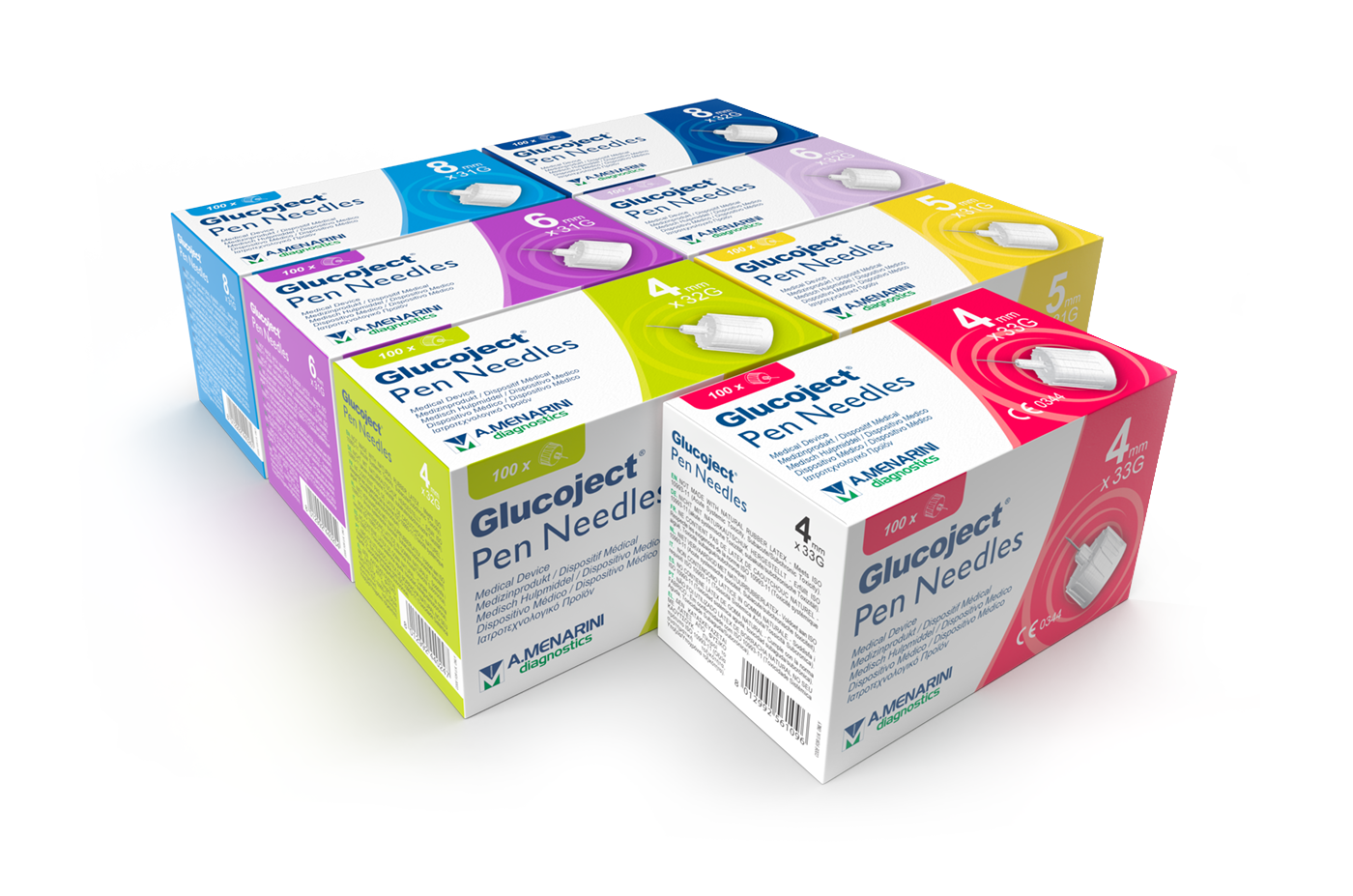
wide range of choice to meet everyone's needs
-
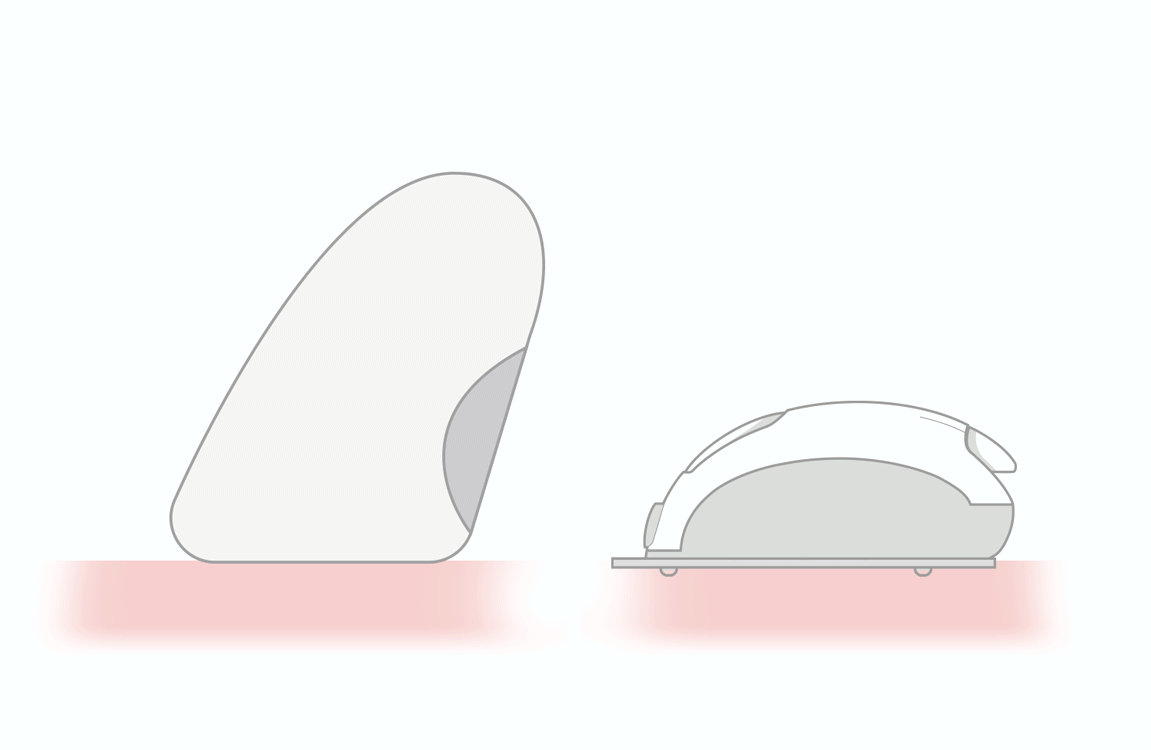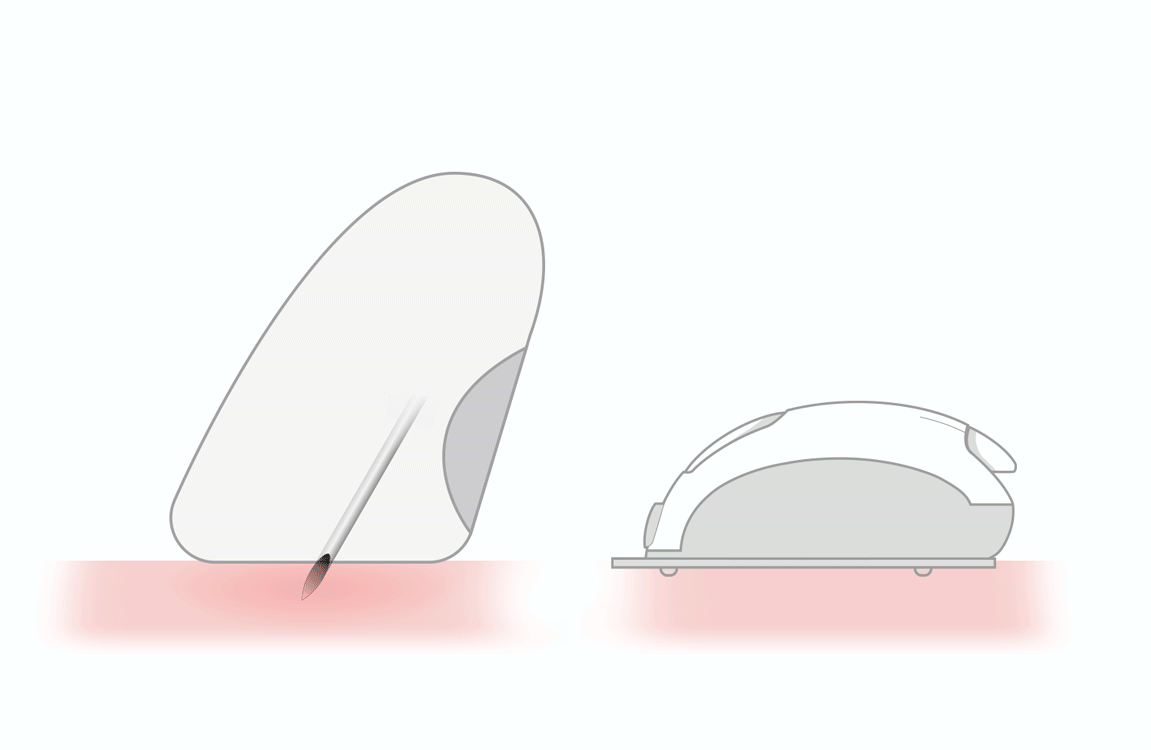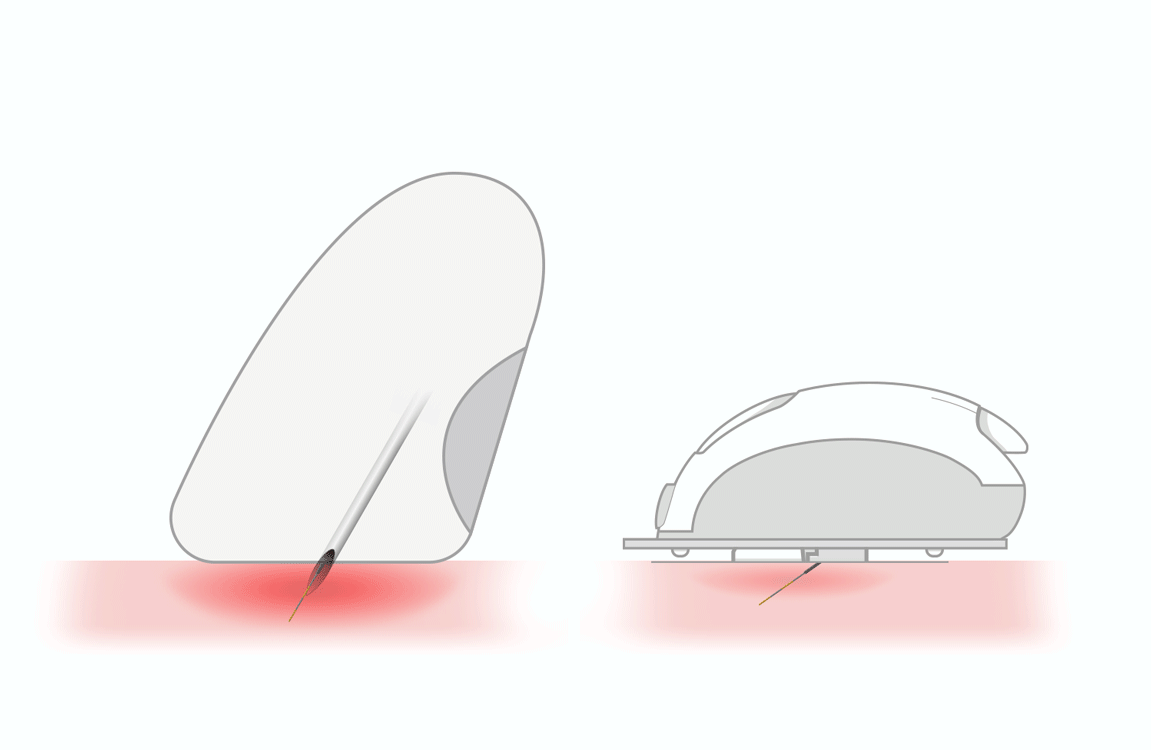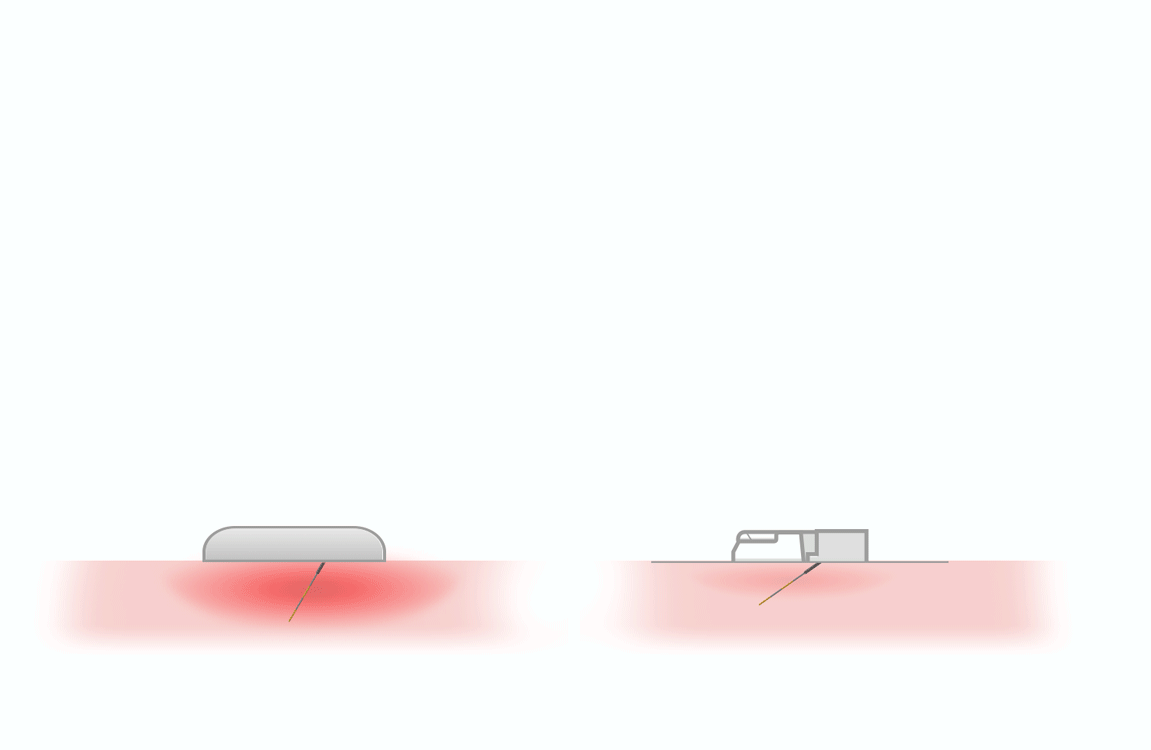
accurate absorption of insulin
by the subcutaneous tissue -
reduces the risk of intramuscular
release of insulin - helps people overcome fear of needles

31G
Glucoject
Pen Needles
31G (0.25mm)
needle lenght:
5mm (3/16”)

31G
Glucoject
Pen Needles
31G (0.25mm)
needle lenght:
6mm (1/4”)

31G
Glucoject
Pen Needles
31G (0.25mm)
needle lenght:
8mm (5/16”)

32G
Glucoject
Pen Needles
32G (0.23mm)
needle lenght:
4mm (5/32”)

32G
Glucoject
Pen Needles
32G (0.23mm)
needle lenght:
6mm (1/4”)

32G
Glucoject
Pen Needles
32G (0.23mm)
needle lenght:
8mm (5/16”)
compatible with
most insulin
pen injectors 10
- screw-in connection designed to fit most insulin pen injectors



4mm x 33G
The new thinner needle designed to support patients with diabetes’ needs. 5,11
A suitable solution for:
- people with diabetes starting insulin therapy
- people with multiple daily injections
-
people with diabetes seeking
greater comfort and with sensitive skin
Technical Specifications
Glucoject Pen Needles

Sterile, single-use pen needles intended for use with
pen injector devices for the subcutaneous injection of drugs.
5 years from sterilization date.
Gamma irradiation.
Meets ISO 10993-11 (Acute Systemic Toxicity, Subacute/Subchronic Toxicity).
• Does not contain natural latex
• Does not contain PVC
• Does not contain phthalates

REFERENCES
1. Luca Leonardi, Mara Viganò, Antonio Nicolucci. Penetration force and cannula sliding profiles of different pen needles: the PICASSO study. Medical Devices: Evidence and Research. 2019:12 311–317.
2. Præstmark KA, Jensen ML, Madsen NB, Kildegaard J, Stallknecht BM5. Pen needle design influences ease of insertion, pain, and skin trauma in subjects with type 2 diabetes. BMJ Open Diabetes Res Care. 2016 Dec 15;4(1):e000266.
3. Siegmund T, Blankenfeld H, Schumm-Draeger PM. Comparison of usability and patient preference for insulin pen needles produced with different production techniques: “thin-wall” needles compared to “regular-wall” needles: an open-label study. Diabetes Technol Ther. 2009 Aug;11(8):523-8.
4. Tandon N, Kalra S, Singh Balhara YT. Forum for Injection Technique (FIT), India: The Indian recommendations 2.0, for best practice in Insulin Injection Technique, 2015. Indian J Endocrinol Metab. 2015 May-Jun; 19(3): 317–31.
5. Frid AH, Kreugel G, Grassi G, et al. New Insulin Delivery Recommendations. Mayo Clin Proc. 2016;91(9):1231-1255.
6. Australian Diabetes Educators Association (ADEA). Clinical Guiding Principles for Subcutaneous Injection Technique. Canberra: 2015.
7. Niels H. Birkebaek, et al. A 4-mm Needle Reduces the Risk of Intramuscular Injections Without Increasing Backflow to Skin Surface in Lean Diabetic Children and Adults. Diabetes Care. Volume 31, Number 9, September 2008.
8. Nadia Tubiana-Rufi, et al. Short Needles (8 mm) Reduce the Risk of Intramuscular Injections in Children Wi t h Type 1 Diabetes. Diabetes Care. Volume 22, Number 10, October 1999.
9. A. K. J. Gradel, et al. Factors Affecting the Absorption of Subcutaneously Administered Insulin: Effect on Variability. Hindawi Journal of Diabetes Research. Volume 2018, Article ID 1205121, 17 pages, https://doi.org/10.1155/2018/1205121.
10. data on file – List of pen injector devices compatible with pen needles manufactured by HTL-Strefa.
11. Valentini M, Scardapane M, Bondanini F, Bossi A, Colatrella A, Girelli A, Ciucci A, Leotta S, Minotti E, Pasotti F, Pesenti A, Rocca L, Sciangula L, Vavassori E, Nicolucci A. Efficacy, safety and acceptability of the new pen needle 33G × 4 mm. AGO 01 study. Curr Med Res Opin. 2015 Mar;31(3):487-92.
* Pen needles are sterile, single use medical devices intended for use with pen injector devices for the subcutaneous injection of drugs.